Cesarean Set Manufacturer,Supplier and Exporter in India

Product Code : EL-SK-11146
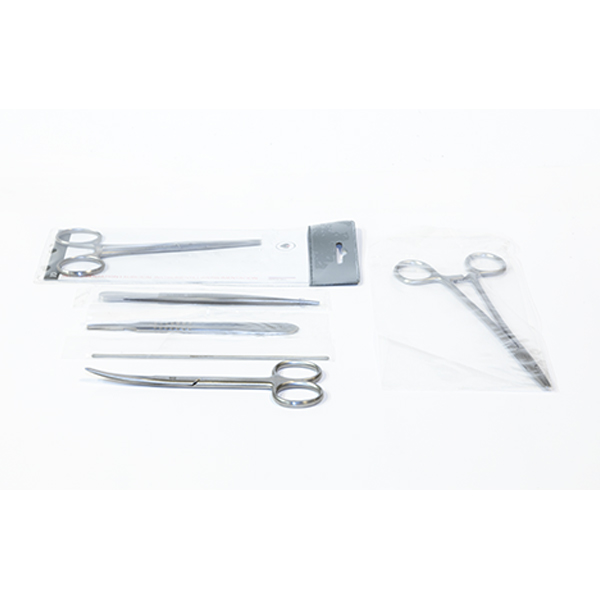
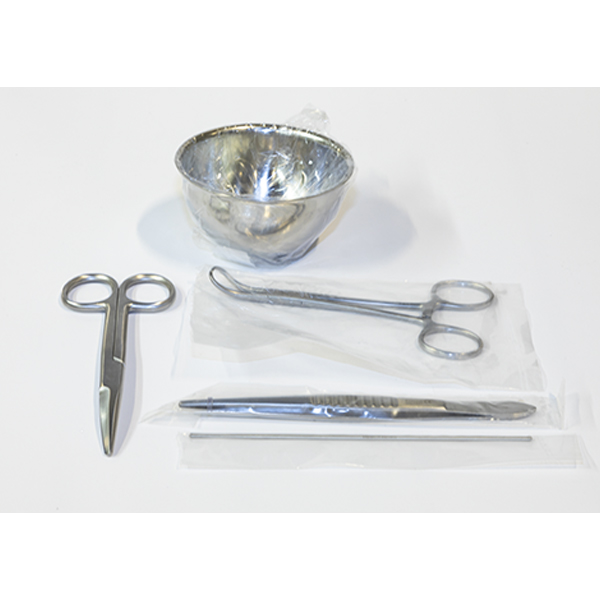
This Cesarean Surgical Instrument Set is a 20-piece
medical-grade kit engineered for obstetric procedures and advanced clinical
simulation. Constructed from corrosion-resistant surgical stainless steel, the
set adheres to ISO standards. Optimized for University-level medical faculties,
Vocational nursing curricula, and Emergency Obstetric and Newborn Care (EmONC)
training, it ensures high-fidelity skill acquisition in laparotomy and neonatal
delivery.
Technical Specifications
|
Tender Specification |
OEM Technical Detail |
|
Material Grade |
Surgical-Grade AISI 410/420 Stainless Steel |
|
Incision Interface |
BP Handles No. 3 and No. 4 (Autoclavable) |
|
Hemostatic Control |
Artery Forceps (Straight/Curved) in 160mm and 200mm
configurations |
|
Tissue Management |
Littlewoods (190mm), Green Armitage, and Toothed/Plain
Forceps (6") |
|
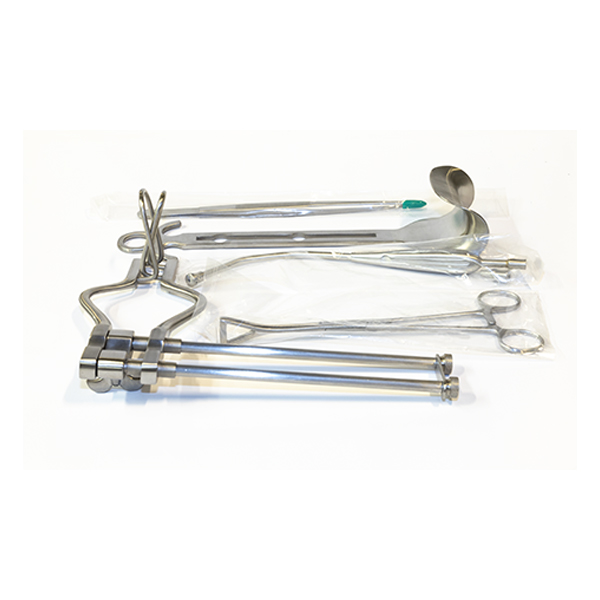
Surgical Retraction |
Dual Langenbeck (45x13mm) and Doyens Retractors |
|
Specialized Obstetric Tools |
Wrigley's Obstetric Forceps and Yankauer Suction Tube |
|
Needle Management |
Mayo Needle Holders (160mm) with precision-milled jaws |
Key Pedagogical Outcomes
- Green
Armitage Forceps: Facilitates specialized training in uterine incision
hemostasis, supporting clinical mastery of postpartum hemorrhage (PPH)
mitigation.
- Wrigley’s
Obstetric Forceps: Enables students to demonstrate safe fetal head
guidance and delivery techniques during low-forceps simulation
(Application/Analysis).
- Doyens
and Langenbeck Retractors: Provides high-visibility access to the
pelvic cavity, essential for teaching anatomical spatial awareness and
tissue protection during a hysterotomy.
- High-Tension
Mayo Needle Holders: Supports the development of advanced suturing
proficiency required for multi-layered uterine closure and fascia repair.
International Logistics & Compliance
Science Lab Export ensures all surgical sets are
prepared for global distribution via export-grade seaworthy crating. Each
shipment includes a Manufacturer’s Authorization Form (MAF), IQ/OQ/PQ
Certification verifying material biocompatibility and sterilization tolerance,
and Technical Manuals in English. The instruments feature a tropicalized
design, rigorously tested for passivation integrity and corrosion resistance in
operational environments reaching 45°C and 90% humidity.